Formaldehyde: An Unwelcome Guest
By HCLI Staff
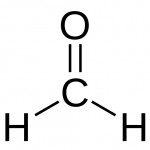
Formaldehyde is one of the most commonly known indoor pollutants. Less familiar might be that natural processes in the atmosphere may contribute up to 90 percent of the total formaldehyde in our environment[1]. As seen in its simple molecular formula H2-C=O (or CH2O, or HCHO, or H2CO) formaldehyde is made of carbon, hydrogen and oxygen, constituting the most basic form of aldehyde, called methanal by its systematic name. Numerous natural and anthropogenic sources contribute to the ubiquitous occurrence of this volatile organic compound (VOC). Even interstellar formaldehyde is widely spread. It was the first polyatomic organic molecule detected in the interstellar medium (ISM), the gas and dust that exist in open space between the stars.[2]
General Properties, Exposure and Impact
At room temperature, formaldehyde is a flammable, colorless gas with a pungent, irritating odor which can be smelled if present in higher concentrations[3], and then should be an alarming indicator for potential health threats. In the environment formaldehyde is broken down within a few hours either by sunlight (photodegradation) or by bacteria inhabiting soil or water (biodegradation)[4]. Within the body it is metabolized and secreted quickly[5]. However, even though it does not accumulate, neither in the environment nor in the body, due to its universal and ongoing distribution Formaldehyde remains to be present permanently, and it depends on its level of air concentration and on individual disposition whether it impairs a person’s well-being.
The exposed body absorbs Formaldehyde easily and mainly through the respiratory tracts and to some extends through the gastrointestinal tracts, i.e. by inhalation and ingestion. For the general population dermal contact, occurring as route of exposure when skin comes into contact with liquids containing formaldehyde, seems to play a minor role, if any.[6]
The normal level of Formaldehyde both outside and inside is less than 0.03 ppm (parts per million parts of air). Usually much lower concentrations are found in rural areas and higher ones in urban areas, and compared to ambient air much higher ratios are found in indoor air[7]. At levels above 0.1 ppm formaldehyde can cause watery eyes, burning sensations in the eyes, nose and throat, nausea, coughing, chest tightness, wheezing, skin rashes, and allergic reactions[8]. Levels of formaldehyde much higher than 0.1 ppm have been found inside some buildings. Factors such as air temperature, humidity, insufficient ventilation, and of course the presence of sources emitting formaldehyde contribute to higher concentrations potentially dangerous. Exposure response among humans varies greatly. Some people may be much more sensitive than others, who seem to have no reaction to the same level.
Occupational exposure
Besides general exposure a large number of people are potentially exposed to higher doses of formaldehyde at their workplace[9]. Workers in factories using formaldehyde-based resins may be exposed to high air concentrations and may also face dermal exposure to liquid forms of formaldehyde. Other groups at higher risk include: dentists, doctors, pathologists, nurses, embalmers, teachers and students who handle preserved specimens in laboratories, veterinarians, and workers in the clothing industry or in furniture factories. [10]
Main Sources
Formaldehyde is an intermediate in the oxidation of methane and other organic compounds (methane cycle). As byproduct of combustion processes on both small and large scale, naturally and manmade, formaldehyde occurs virtually everywhere; e.g. in cells of all organic life forms including humans, in fuel driven engines, in the troposphere, or interstellar. Due to its major role as basic building-block molecule for a wide range of products of today’s society, formaldehyde is widely spread in manufacture lines and is emitted by building material and consumer products. Especially inside buildings human activities and manmade products are dominating sources of higher formaldehyde air concentrations. Although ambient air may hold 90% of all formaldehyde, mean open air concentrations are significantly lower because here formaldehyde scatters throughout a much larger space.
Natural Sources
Formaldehyde derives as precursor from many natural processes and further decomposes into carbon monoxide and dioxide, hydrogen and water. Forest and bush fires release large amounts of formaldehyde. The oxidation of hydrocarbons in the troposphere caused by sunlight generates formaldehyde. Terpenes and isoprene produced and emitted by plants, per example, react with hydroxyl radicals leading to formaldehyde. As endogenous chemical emerging by metabolic processes formaldehyde occurs in minute amounts in nearly all forms of life.
Anthropogenic Sources
Manufacture and road traffic
Besides production facilities, where formaldehyde is either produced or technologically applied, the by far largest primary anthropogenic source are exhaust emissions of fuel driven vehicles. Both formaldehyde production processes[11] and automotive traffic are growing worldwide year by year. While critical concentrations of formaldehyde in and around industrial facilities are restricted locally, formaldehyde emission caused by on-road traffic is widely spread around the world. Since highest densities of road traffic take place in areas of high densities of population, the highest levels of on-road automotive releases are found where most people live. Since transportation never stops, decomposed Formaldehyde is replaced continuously, and obviously levels of exposure in densely populated areas remain comparatively high.
Indoor air at higher levels
Naturally the quality of indoor air cannot be better than the quality of ambient air surrounding building enclosures. In fact, additional sources of formaldehyde and limited airflow generally elevate indoor levels of air concentration. Studies in various countries have shown that indoor air contains substantially more Formaldehyde than outdoor air.
Indoor combustion processes
Unwanted combustion spills from boiler or water heaters (gas or oil), from wood stoves and fireplaces (wood), or from other fuel burning devices. Other combustion processes, such as certain cooking activities[12], in some cases tobacco consumption[13], or candle and incense burning, may add to indoor formaldehyde contamination.
Off-gassing
The off-gassing from building materials (e.g. pressed wood, carpets, adhesives), which is most relevant in new or lately renovated spaces, and the off-gassing from a wide range of consumer products often dominate indoor sources of formaldehyde.
Example: particle boards
New built-in or mobile furniture made of particleboard are known for emitting comparatively high doses of formaldehyde. Kitchen cabinetry is one of the classical cases. The newer those products are the more formaldehyde they release. The first weeks, in some cases up to a year or longer, special attention must be paid to them and frequent ventilation of rooms where they are placed is necessary[14]. In extreme cases, when particleboard with high formaldehyde ratios[15] has been installed, formaldehyde air contamination can last for years and a complete and permanent removal could be the only way of eliminating the problem.
Mobile homes
The manufacturing process of travel trailers and mobile homes, a major housing facility in the US[16], involves the use of particularly large quantities of formaldehyde catalyzed resins needed for the production and assembly of particle board, fiberboard, plywood, surface coating, and foam insulation. Consequently, in many trailers, besides other pollutants, dangerous levels of formaldehyde fumes are detected[17].
Example: contaminated FEMA trailer
One recent prominent example of alarming formaldehyde levels in residential indoor air is the FEMA trailer incident. A large number of trailers and mobile homesprovided by the US Federal Emergency Management Agency (FEMA) for the housing of displaced victims of the hurricanes Katrina and Rita[18] have been found contaminated by formaldehyde in an unusually high average level of 0.77 ppm[19], and with many levels higher than this average, even more than two years after manufacturing[20]. After a number of occupants complained about respiratory distress such as asthma, sinus infections and bronchitis, nosebleeds, skin rashes, burning eyes, and persistent headaches, random samples of 519 travel trailers and mobile homes were tested between December 2007 and January 2008. Formaldehyde catalyzed resins of composite wood and plywood panels are believed to be the primary source of the massive formaldehyde emission in FEMA’s temporary housing units.
Sadly but not surprisingly, the manufacturer who distributed those trailers to FEMA knew about their hazardous formaldehyde levels, yet for the sake of “good” business ignored them.[21] Even though lawsuits have been filed against FEMA in this case, and despite FEMA’s promise it would work aggressively to relocate all residents of the temporary housing as soon as possible, a similar but smaller case occurred when again FEMA provided trailers for people displaced by the Iowa floods of June and July 2008[22].
Wide range of products contain Formaldehyde
According to the American Chemistry Council, “every day, people benefit from products that contain formaldehyde. This chemical is a critical, commercially valuable, and basic building block in our modern society. … Products derived from formaldehyde have an extremely broad role in the economy and provide many benefits to the public. In many instances … few compounds can replace it as a raw material without reducing performance and making the final product more expensive.” [23]
Indeed, formaldehyde is not only ubiquitous in nature but also appears in countless products. The items listed below may contain and release formaldehyde or formaldehyde derivates[24]. Doses for one type of product can vary from zero or insignificantly low to rarely alarmingly high[25]. However, regardless how low the input of a single source might be, in worst case scenarios an unfavorable mix of primary and secondary sources can build up to an unwanted overall load of exposure. In general, BFGC recommends the examination of goods concerning potentially harmful substances before purchasing.
- Formaldehyde is used in resins, largely needed for the manufacturing of pressed wood products, such as furniture, cabinets and building material made from particleboard, medium density fiberboard, and plywood.
- Formaldehyde is an essential intermediate in the production process of many industrial chemical compounds, which in turn are used for manufacturing a great variety of raw and final products, such as polyurethane and polyester plastics, synthetic resin coating, synthetic lubricating oils, plasticizers, surface coatings, explosives, detergents, soft and rigid foams, and adhesives.
- The polymerization of formaldehyde leads to polyacetal plastic components found in automobiles, in airplanes, and in electronic equipment, such as audio, video or computer devices.
- Formaldehyde is the basis for products, such as dyes, tanning agents, precursors of dispersion and plastics, extraction agents, crop protection agents, animal feed, perfumes, vitamins, flavorings and drugs.[26]
- As preserving and disinfecting agent formaldehyde is directly used in human and veterinary drugs including vaccines, for disinfecting hospitals, for preserving and embalming biological specimens and cadaver, and in dentistry.
- Furthermore it is used as antimicrobial agent in cosmetics, such as soap, shampoo, hair preparation, shaving cream, deodorant, lipsticks, lotion, make-up, toothpaste, mouthwash and breath fresheners, and nail polish and hardener.[27]
- As anti-corrosion agent formaldehyde is part of mirror finishing and electro-plating, of the electro-deposition of printed circuits, and of some photographic film developing processes.[28]
- In the textile industry formaldehyde is used in crease-resistant finishes and in preservatives against fungal and other microbial decay, and against insects.
- The paper industry applies formaldehyde in special treatments and coatings for some products, such as paper towels, paper money, and other special papers or cardboards.
- In agriculture formaldehyde is applied as part of selected pesticides, in some fertilizers, as preservative for fodder, as a disinfectant, and in fumigation of products such as grain.
- Although in small amounts, besides its natural occurrence in some food items, formaldehyde can be added to final fresh and preserved food products as well.[29]
- Besides the above-mentioned commercial products and sanitary articles, here are some further popular consumer goods known for possibly or certainly containing formaldehyde: paint, lacquers, varnishes, wallpaper, cardboard and paper products, carpeting, durable press fabrics such as some curtains, sheets and certain clothing, fabric softeners, dishwashing liquids, shoe-care agents, fur and leather products, carpet cleaners, glues, adhesives, cigarettes, garden fertilizers, ink, decorative laminates.
Regulation of Formaldehyde
In many countries the use of formaldehyde is regulated. Often different agencies define different values for acceptable exposure limits for different purposes. In the US, for example, the EPA[30] has determined the exposure to formaldehyde in drinking water not expected to cause any adverse effects in a child at concentrations of 10 mg/L for 1 day or 5 mg/L for 10 days; the EPA has also set the general possible life time exposure not expected to cause any adverse effects to 1 ppm; The OSHA[31] has set the occupational exposure limit to an average of 0.75 ppm for 8-hour workdays and 40-hour workweeks; and the HUD[32] has set standard emission levels in manufactured housing at less than 0.2 ppm for Plywood and 0.3 ppm for particle board in order to provide a maximum ambient level of 0.4 ppm in manufactured housing.
In many cases existing surveys or studies do not cover the complete range of situations a defined exposure limit is set up for. This often applies to both the quality and the quantity of data. Information gained by animal experiments cannot be extrapolated to humans straight-line. Another problem might be posed by data collected not recently enough to be projected directly to current circumstances. And finally, different models of interpretation applied to the same pool of data can lead to different results. All this explains the sometimes wide range of numbers for the very same type of exposure limit recommended by the various institutions.
Government agencies that must consider a set of frequently contradicting viewpoints – ranging from health protection of individuals and the general public up to the competitiveness of companies, complete industrial sectors, and even the economy of a country as a whole – may conclude guidelines for exposure limits in a different way compared to a team of independent scientists devoted to the prevention of any possible health risk.
History of formaldehyde regulation
Frequently the knowledge backing up assumptions is incomplete or inaccurate or influenced by subjective prejudices. Newly acquired data could lead to revised insights. The history of the evaluation of health effects of formaldehyde is one of the many examples illustrating how science slowly finds increasing evidence for harmful long-term implications of doses lower than previously thought. Since commercial formaldehyde production began in 1889, great numbers of people have been exposed to it unregulated for more than 80 years. The increasing number of complains in the 1970s and 1980s about health problems caused by consumer products in homes containing fairly huge amounts of formaldehyde prompted health administrations to release various guidelines which not always, however, have been translated into legislation. At that time concerns that Formaldehyde could cause cancer arose when studies showed high levels of exposure, 6 – 15 ppm, induces nasal cancer in laboratory rats. Nevertheless, as these extreme exposure levels are not found in human environments, for the next two decades epidemiological studies failed to provide enough evidence for a potential carcinogenic risk to humans, and formaldehyde was classified first 1987 by the US EPA, and later 1995 by the ICAR[33] as “probably carcinogenic to humans” (Group 2A). In 2004, sufficient epidemiological evidence of nasopharyngeal cancer caused among formaldehyde-exposed workers[34] has led the ICAR to finally reclassify formaldehyde as “carcinogenic to humans” (Group 1). A link between occupational formaldehyde exposure and the development of myeloid leukemia documented in recent studies[35] seems to back up the reclassification.
This judgment promoted by the WHO is, however, controversial and has not yet caused action among national health agencies.[36] A recognition of formaldehyde as carcinogenic agent on governmental level would have sweeping consequences – in the EU, per example, according to existing legislation up to a severely restricted consumer exposure and a higher risk management at workplaces handling formaldehyde.
Current legal regulation on formaldehyde is limited to most urgent aspects of its use. For occupational formaldehyde exposure, 1987 OSHA reduced the permitted limit for 8-hour workdays from 3 ppm to 1 ppm, and amended this value again 1992 down to 0.75 ppm. In the field of consumer products a bill was passed in US congress 2010, to be in effect by January, 2013, restricting formaldehyde emission from plywood, particle board and medium density fiberboard to a maximum of 0.09 ppm.[37] In the EU generally the maximum concentration of formaldehyde allowed in finished products is 0.2% and any product holding a concentration of more than 0.05% has to be labeled with a warning that the product contains formaldehyde.
Conclusion
Generally, it is widely agreed that formaldehyde occurs in doses well below levels critical for human health. Higher air concentrations are found at workplaces related to the production or application of formaldehyde. The major exposure route for formaldehyde is inhalation from indoor sources. Considering the average time people spend inside, as well as the higher levels of indoor air concentration due to emitting building materials and consumer products, indoor exposure to formaldehyde contributes up to 98% to the average overall exposure. In some indoor locations concentration levels approach limits associated with signs of eye and respiratory tract sensory irritation.
Factors affecting formaldehyde emission
- Indoor formaldehyde air concentrations vary according to following circumstances[38]:
- The age of the building or the time passed since remodeling – since released formaldehyde degrades quickly, usually older buildings contain less formaldehyde.
- The air exchange rate – the more indoor spaces are ventilated the less formaldehyde vapor can build up in them.
- Air temperature and relative humidity – the higher both parameters are the more formaldehyde might be released as resins and other formaldehyde binding compounds are breaking off.
- The season – because outdoor climate influences indoor temperature and humidity, in colder seasons windows are kept close, indoor heating devices can raise levels of formaldehyde, and lower outside temperatures lead to the fall out of condensation water inside. In warmer regions indoor formaldehyde levels are generally lower.
What can be done?
While it seems impossible to completely avoid exposure to materials and products releasing formaldehyde, the following measures can help reducing the level of formaldehyde air contamination around you:
- First and most effective of all: Increase the air exchange rate of enclosed rooms, including vehicles, by opening doors and windows, and in rooms with no direct wall openings to the outside operate automated air exhausters. This advice does not only relate to formaldehyde emission but generally to improving indoor air quality.
- Avoid unnecessarily high room temperatures and reduce indoor humidity.
- Particle wood products made with formaldehyde resins are often a significant source of formaldehyde in homes. If you cannot avoid buying or using such products, as in cabinetry, furniture or flooring, look for certified eco-labels, such as the Blue Angel[39] in Germany, or the Green Seal in the US. Here certain ANSI[40] grades stamped on panels specify lower formaldehyde emission levels. So called “exterior-grade” pressed wood contains less formaldehyde.[41]
- Choose furniture or cabinets containing a higher percentage of laminated or coated pressed wood panels with sealed edges. They generally emit less formaldehyde than openly exposed wood panels.
- Before buying any consumer product check for harmful substances – not only for formaldehyde, of course.
- Do not use unvented or insufficiently vented indoor heaters.
- Strictly limit or completely avoid the use of formaldehyde containing cleaning and disinfecting supplies.
- Avoid cosmetics containing formaldehyde. Amounts of 0.05% and higher must be labeled.
- Do not smoke. The smoke of one cigarette can release up to 0.3 mg formaldehyde.
- Wash durable-press fabrics before use.
Endnotes
[1] WHO, 2002, Concise International Chemical Assessment Document: Formaldehyde
[2] Snyder et al., 1970, Microwave Detection of Interstellar Formaldehyde: “Interstellar formaldehyde (H2CO) has been detected in absorption against numerous galactic and extragalactic radio sources by means of the 111-110 ground-state rotational transition at 4830 MHz. The absorbing regions often correspond in velocity with 18-cm OH features. H2CO is the first organic polyatomic molecule ever detected in the interstellar medium and its widespread distribution indicates that processes of interstellar chemical evolution may be much more complex than previously assumed.”
[3] Most people recognize Formaldehyde in the air at a level of about 0.25 ppm (0.3 mg/m3). (DISU Mailbox Datenblatt Formaldehyde, DISU, Germany, 2007)
[4] Source: US Agency for Toxic Substances and Disease Registry (ATSDR), Toxicological Profile for Formaldehyde (1999); Lu et al (2010)
[5] The half life of inhaled Formaldehyde in the body is 10-15 minutes. A small part of it might be built into endogenous substances. (DISU Mailbox Datenblatt Formaldehyde, DISU, Germany, 2007)
[6] Environment Canada, 2001, Priority substances list assessment report, formaldehyde: “Available data on effects following ingestion or dermal exposure to formaldehyde are limited.”
[7] Examples of formaldehyde concentrations: 0.0002 – 0.006 ppm in rural and suburban outdoor air, 0.0015 – 0.047 ppm in urban outdoor air, 0.020 – 4 ppm in indoor air (ATSDR, Public Health Statement Formaldehyde, 2008)
[8] US Consumer Product Safety Commission (CPSC), An Update on Formaldehyde, 1997 Revision
[9] No recent statistics are available. In a study by Kauppinen et al., 2000, CAREX, 2003, approximate numbers of workers exposed to formaldehyde above background levels (0.1 ppm) in the European Union 1990-93 in major industry sectors have been estimated. All industries combined presented a total of 971,000 people. For the US the National Institute for Occupational Safety and Health (NIOSH) survey (National Occupational Exposure Survey (NOES) 1981-1983) has statistically estimated that 1,329,322 workers (441,902 of them female) are potentially exposed to formaldehyde. The NOES Survey does not include farm workers.
[10] ATSDR, Public Health Statement Formaldehyde, 2008
[11] The USA, Western Europe, China and Japan produce and consume the largest quantities of 37% formaldehyde solution, the most typical commercial formaldehyde raw product.
[12] “Data from several studies indicate that various cooking activities may contribute to the elevated levels of formaldehyde sometimes present in indoor air (Health Canada, 2000). In recent work from the USA, the emission rate of formaldehyde from meat charbroiling over a natural gas-fired grill in a commercial facility was higher (i.e., 1.38 g/kg of meat cooked) than emission rates of all other VOCs measured except for ethylene (Schauer et al., 1999).” (CICADS, 2002, Formaldehyde)
[13] Cigarettes contain remarkable loads of Formaldehyde. “A range of mainstream smoke emission factors from 73.8 to 283.8 µg/cigarette was reported for 26 US brands, which included non-filter, filter, and menthol cigarettes of various lengths (Miyake & Shibamoto, 1995) ” Nevertheless “ETS (environmental tobacco smoke) does not increase concentrations of formaldehyde in indoor air, expect in areas with high rates of smoking and minimal rates of ventilation (Godish, 1989; Guerin et al. 1992).” (CICADS, 2002, Formaldehyde)
[14] According to a US Environmental Protection Agency (EPA) study a new home measured 0.076 ppm formaldehyde when brand new and 0.045 ppm after 30 days.
[15] In the US, per example, recent legislation, to be in effect by January 2013, is going to limit the emission of formaldehyde from composite wood products to a maximum of 0.09 ppm. In the past, however, composite wood of much higher emission has been used, and there is no guarantee such material is still occasionally deployed.
[16] Approximately 8 million US citizens live in trailers and mobile homes.
[17] In addition to off-gassing building materials further typical sources of formaldehyde in trailers are insufficient air circulation of HVAC units and indoor emissions of gas burning cooking devices during times and seasons of restricted air ventilation.
[18] Hurricane Katrina of the historic 2005 Atlantic hurricane season was the costliest natural disaster, as well as one of the five deadliest hurricanes, in the history of the United States. August 29 Katrina hit the Gulf coast from central Florida to Texas. New Orleans, southeast Louisiana, and the coastal areas of Mississippi were affected most severely. Hurricane Rita hit again the Gulf cost of Texas and Louisiana just four weeks later, September 23, and was the fourth-most intense Atlantic hurricane and the most intense tropical cyclone in the Gulf of Mexico ever recorded. Some 143,000 trailers were used as emergency housing units following the two storms.
[19] Normal levels of formaldehyde are 0.1 to 0.2 ppm
[20] US CDC: Interim Findings on Formaldehyde Levels in FEMA-Supplied Travel Trailers, Park Models, and Mobile Homes from the Centers for Disease Control and Prevention, February 29, 2008. FEMA started to supply trailers 2006.
[21] “In July 2008, officials for Gulf Stream Coach, Forest River, Keystone RV, and Pilgrim International testified before the House Oversight and Government Reform Committee. They admitted that they knew the FEMA trailers they made for the hurricane victims contained unsafe levels of formaldehyde.” (Source: Product Liability Law Blog, December 9, 2009, in: Thousands of FEMA Trailer Claims Filed by Victims of Hurricane Katrina and Rita)
[22] Rep. Bruce Braley, 2008 press releases: “Washington, DC – Rep. Bruce Braley (D-Iowa) released the following statement today after reports that unsafe levels of formaldehyde have been detected in temporary FEMA housing in Iowa: “It is disturbing and unacceptable that temporary housing provided by the agency responsible for helping people in times of emergency could be making them ill,” Braley said. “Iowans affected by this year’s unprecedented floods and tornadoes should be able to have confidence that FEMA will be helpful to them during this difficult time, not harmful to their health. …”
[23] American Chemistry Council, Inc. at www.formaldehydefacts.org, retrieved March 2011
[24] Taken partly from IARC monographs, volume 88, 2006;
[25] In developed countries under normal circumstances nowadays critical doses of formaldehyde in residential and other buildings not engaged in formaldehyde processing are rare and do not last long enough in order to impose verifiable health risks. In older buildings, constructed before formaldehyde has been regulated, formaldehyde from once highly contaminated building material has most likely degenerated by now. In those buildings semi volatile pollutants (e.g. PAH) remain more of a problem today. However, the recent FEMA trailer incident described above indicates potential risks of hazardous indoor formaldehyde exposure cannot be ruled out.
[26] WHO, 1989; Reuss et al., 2003
[27] Cosmetic Ingredient Review Expert Panel, 1984; Reuss et al., 2003
[28] Reuss et al., 2003
[29] Besides naturally low levels in some food (e.g. fruits), formaldehyde in fertilizers, fumigants, or preservatives may add additional exposure. “The Food and Drug Administration (2003) in the USA identifies formaldehyde: as a secondary direct food additive that is permitted in food for human consumption; for use as a preservative in defoaming agents; as an indirect food additive for use only as a component of adhesives; as an indirect food additive for use only as paper and paperboard components; as an indirect food additive for use only as a preservative in textile and textile fiber polymers; as an indirect food additive for use as an adjuvant in animal glue; and, under specified conditions, as an animal drug and in the manufacture of animal feeds.” (IARC monographs, volume 88, 2004)
[30] EPA: Environmental Protection Agency
[31] OSHA: Occupational Safety and Health Administration
[32] HUD: Department of Housing and Urban Development
[33] IARC: International Agency for Research on Cancer, WHO
[34] 1994 follow-up of the National Cancer Institute (NCI) cohort study of formaldehyde-exposed workers (Hauptmann et al., 2003; 2004)
[35] Zhang et al., 2010, Formaldehyde and Leukemia: Epidemiology, Potential Mechanisms, and Implications for Risk Assessment; Zhang et al., 2008, Formaldehyde exposure and leukemia: A new meta-analysis and potential mechanisms
[36] Up to now the WHO itself did not issue the substantiation of her reclassification in 2004, yet.
[37] US Congress, Formaldehyde Standards for Composite Wood Products Act, 2010
[38] According to: WHO guidelines for indoor air quality: selected pollutants, 2010
[39] The Blue Angel (Blauer Engel) certificate, introduced in Germany 1978, was the first environmental label worldwide.
[40] ANSI: American National Standards Institute
[41] Exterior-grade pressed wood is made with phenol resins instead of urea resins, and therefore emits less formaldehyde.
Copyright © 2011 Hemato-Centric Life Institute